Platinum is an inert metal and does not participate in the electrode reaction itself, so it is the first choice for the counter electrode. One of the important parameters of the counter electrode is its surface area. The counter electrode must have a large enough area to support the current conduction generated on the working electrode. Platinum electrodes include platinum sheet electrodes, platinum wire electrodes, and platinum mesh electrodes.
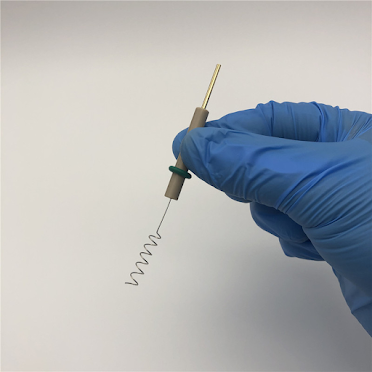
The platinum wire electrode has a relatively small area ,which is suitable for use in combination with a disc-shaped working electrode. The platinum wire electrode has a variety of shapes and structures, which can be bent according to the length of the platinum wire to be used with various types of electrochemical cells. The insulating rod material of the platinum wire electrode can be PTFE or PEEK rod.
As the working electrode undergoes an oxidation or reduction reaction, a gas evolution reaction or a reverse reaction of the working electrode reaction may occur on the counter electrode. Therefore, in some cases it is necessary to separate the working electrode from the counter electrode. The best way to reduce the interference of the reaction on the counter electrode on the working electrode is to use sintered glass, porous ceramics or ion exchange membranes to isolate the solution in the two electrode regions.



Comments
Post a Comment