Glassy carbon electrode is one of the most widely used working electrodes, and it is a better inert electrode. Glassy carbon electrode has many advantages such as good conductivity, high hardness, high finish, high hydrogen overpotential, and wide polarization range. Its chemical properties are very stable, and it can be used as an inert electrode for direct anodic dissolution testing, and voltammetric determination of cathodes and variable valence ions. Using glassy carbon electrodes as the substrate, chemically modified electrodes can also be prepared.
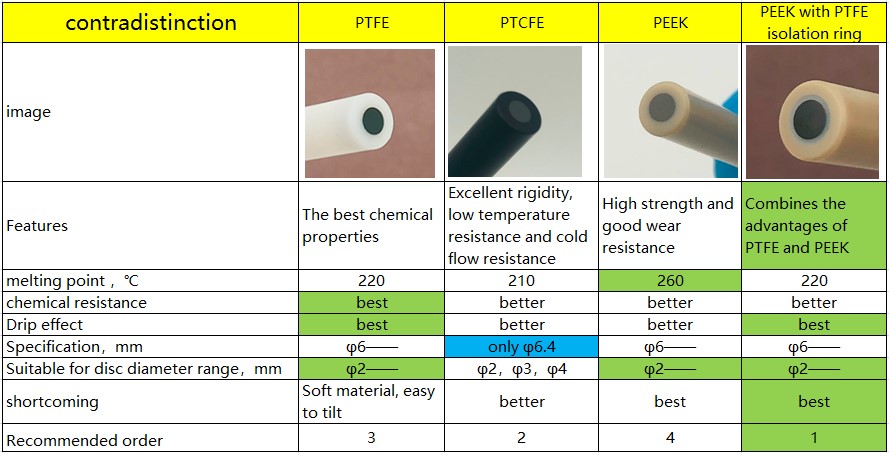
There are four types of insulating rods for glassy carbon electrode jackets.
Glassy carbon electrodes are divided into straight type, L type, T type and detachable type. The straight glassy carbon electrode material faces vertically downwards, and the research material is coated on the glassy carbon material surface for testing. The reference electrode is matched with a curved luggin capillary salt bridge close to the material surface of the glassy carbon electrode, which can effectively reduce the R drop and improve the test effect.
Check details: Glassy Carbon Electrode Straight Type PTCFE Rod φ3mm - Dekresearch


Comments
Post a Comment