According to the test requirements, you can use the following content as a reference for selecting reference electrode.
Different research systems can choose different reference electrodes:
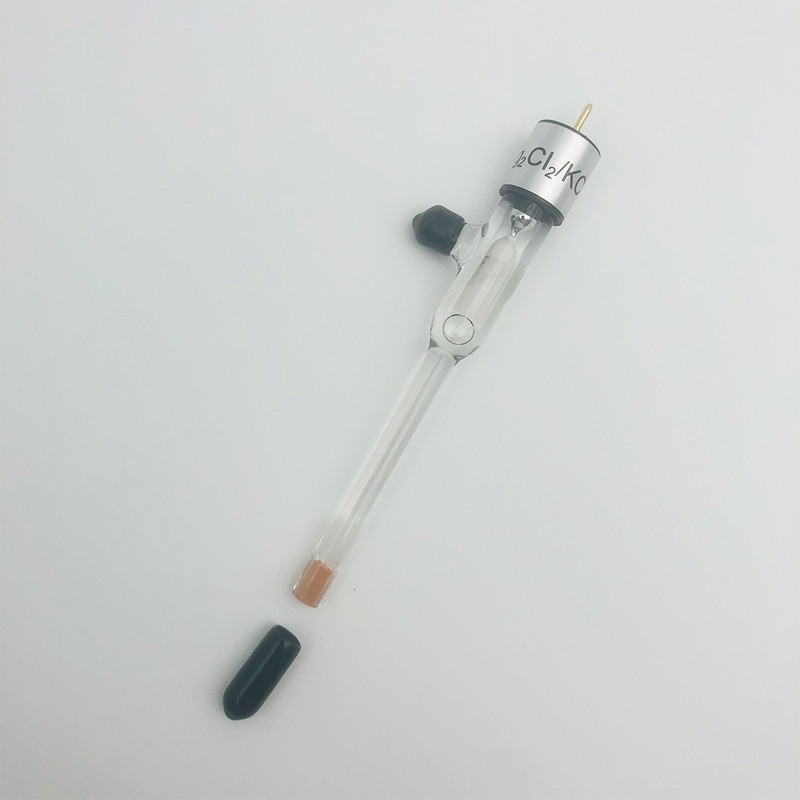
Neutral system: saturated calomel electrode or Ag/AgCl reference electrode
Alkaline system: Hg/HgO reference electrode
Acidic system: Hg/Hg2SO4 reference electrode
Non-aqueous system: Non aqueous Ag+ reference electrode
Compared with other reference electrodes, Ag/AgCl reference electrode is more sensitive to light.At the same time, the silver chloride electrode is also less affected by temperature than other types of reference electrode.
If you choose calomel electrode, Hg2/HgSO4 electrode or Hg/HgO electrode, you can add a salt bridge under the electrode to protect the reference electrode and minimize the potential affected by temperature.
Mercury Oxide Reference Electrode Hg/HgO PTFE Rod 6 * 60 mm is a good choice for the Alkaline system.
you can find all the reference electrodes from here: https://www.dekresearch.com/dekresearch-electrodes/-Reference+Electrodes--------



Comments
Post a Comment